How To Find Moles With Molarity And Volume
This molarity calculator is a tool for converting the mass concentration of any solution to tooth concentration (or recalculating grams per ml to moles). Y'all tin can also calculate the mass of a substance needed to achieve a desired molarity. This article will provide you lot with the molarity definition and the molarity formula.
To understand the topic as a whole, you will want to learn the mole definition, read a paragraph well-nigh the molarity units, equally well as read a comparing of 2 misleading concepts: molarity formula vs molality formula. What is more, we prepared for yous some interesting examples of tooth solutions and a short step-by-step tutorial of how to calculate molarity of a concentrated solution.
At the stop, you can learn the titration definition and discover how to find the molar concentration using the titration procedure, which may exist helpful when carrying out titrations!
Molar concentration – an introduction
When you lot look around, even if you're sitting at home, you volition discover many different objects. The majority of these materials are non pure. They are, in fact, mixtures.
Mixtures consist of a collection of different compounds. Occasionally, the number of elements may exist quite loftier, or sometimes quite low, but equally long as at that place is more than one element in an object, it is a mixture. Orange juice in your glass, a cup of tea, detergents in the bath or milk – all these substances are mixtures.
Mixtures are not limited to just liquids though, solids and gases can both be mixtures; even biological organisms are very complex mixtures of molecules, gases, and ions dissolved in h2o.
In chemistry, there are two types of mixtures:
-
Homogeneous mixtures – Components are uniformly distributed throughout the mixture, and there is simply one phase of matter observed. They are besides known as solutions and may occur in the solid, liquid or gaseous state. Information technology is non possible to simply separate the mixture components, only no chemical change has occurred to any of the components. Examples: saccharide water, dishwashing detergent, steel, windshield washer fluid, air.
-
Heterogeneous mixtures – Components of the mixture are not uniformly distributed and may accept regions with dissimilar backdrop. Different samples of the mixture are not identical. At least two phases are ever present in the mixture, and it'south usually possible to physically divide them. A few examples of such substances: claret, concrete, water ice cubes in cola, pizza, the Pacific Sea.
Concentration is one of the nearly well known and about important parameters for anybody who works with whatever chemic substances or reactions. Information technology measures how much of a substance is dissolved in a given volume of solution.
Chemists use many different units for describing concentration. However, the term molarity, likewise known as molar concentration, is the most mutual way of expressing the concentration. When the reactants (compounds) are expressed in mole units, it allows them to exist written with integers in chemical reactions. This helps to easily piece of work with their amounts. First, let's take a closer expect at what is the mole, so we tin can move on later to detect what is molarity.
Mole definition
The mole is the SI unit of measurement for the amount of substance. The current definition was adopted in 1971 and is based on carbon-12. It says:
"The mole is the amount of substance of a organization which contains as many elementary entities as there are atoms in 0.012 kilograms of carbon-12; its symbol is "mol". When the mole is used, the elementary entities must exist specified and may exist atoms, molecules, ions, electrons, other particles, or specified groups of such particles."
It follows that the molar mass of carbon-12 is exactly 12 grams per mole, M(¹²C) = 12 g/mol. The word "substance" in the definition should specify (be replaced with the name of) the substance concerned in a particular application, e.chiliad., the amount of chloride (HCl) or the corporeality of carbon dioxide (CO₂). It is crucial to e'er give a precise specification of the entity involved (every bit noted in the second part of the mole definition). This should exist done by providing the empirical chemical formula of the compound involved.
Co-ordinate to the newest conventions (constructive as of the 20th May 2019), the mole definition is that a mole is the corporeality of a chemical substance that contains exactly half dozen.02214076 × 1023 particles, such equally atoms, molecules, ions etc. That number is known equally Avogadro's constant. Its symbol is NA or L. Using the Avogadro number provides a convenient fashion of considering the weight of substance and the theoretical yield of chemical reactions. Moles allow you to straight read weight from the periodic table (due east.g., 1 mole of N₂ is 28 m or 1 mole of NaCl is 58.5 1000).
Nosotros can link the number of entities 10 in a specified sample – North(X), to the moles of X in the aforementioned sample – due north(X), with the relation: n(Ten) = Northward(X)/NA. North(10) is dimensionless, and north(X) has the SI unit mole.
What is molarity?
And so yous are not confused with similar chemical terms, go on in listen that molarity ways exactly the same every bit molar concentration (Thousand). Molarity expresses the concentration of a solution. It is defined equally the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!).
concentration = number of moles / volume
Molarity formula
The following equation allows you to observe the molarity of a solution:
molarity = concentration / molar mass
The concentration denotes the mass concentration of the solution, expressed in units of density (commonly m/50 or 1000/ml).
Molar mass is the mass of ane mole of the solute. Information technology is expressed in grams per mole. It is a constant property of each substance – for case, the molar mass of h2o is approximately equal to eighteen yard/mol.
Our calculator can also find the mass of substance you demand to add to your solution to obtain a desired tooth concentration, according to the formula:
mass / volume = concentration = molarity * molar mass
where mass is the mass of solute (substance) in grams, and volume is the full volume of solution in liters.
Molarity has many applications. One of them is the calculating the solution dilution.
Molarity units
The units of tooth concentration are moles per cubic decimeter. They are noted as mol/dm³ as well every bit G (pronounced "molar"). The molar concentration of solute is sometimes abbreviated by putting foursquare brackets effectually the chemic formula of the solute, e.g., the concentration of hydroxide anions tin be written every bit [OH⁻]. In many older books or articles, yous can discover dissimilar units of molar solutions – moles per liter (mol/l). Retrieve that ane cubic decimeter equals to one liter, so these two notations express the same numeric values.
Formerly, chemists used to give concentrations equally the weight of solute/volume. Nowadays, since mole has become the well-nigh mutual way of quoting the quantity of a chemical substance, molarity is commonly used instead.
Note that molarity might be quite ofttimes dislocated with the term molality. Molality is commonly written with lower case k, while molarity (what was mentioned above) with an capital 1000. We explain the difference between these two in a paragraph below.
Molarity too plays a significant role in calculating the ionic strength of a solution.
How to calculate molarity
- Choose your substance. Let's assume that information technology is the hydrochloric acid (HCl).
- Discover the molar mass of your substance. For the muriatic acid, information technology is equal to 36.46 g/mol.
- Decide on the mass concentration of your substance – you can either input information technology directly or fill in the boxes for substance mass and solution volume. Let's assume that you have five k of HCl in a one.2 liter solution.
- Convert the expressions above to obtain a molarity formula. As
mass / book = molarity * tooth mass, and thenmass / (volume * tooth mass) = molarity. - Substitute the known values to summate the molarity:
molarity = v / (one.ii * 36.46) = 0.114 mol/l = 0.114 M. - You can also use this molarity figurer to discover the mass concentration or molar mass. Simply type in the remaining values and watch it do all the piece of work for you.
Molarity vs molality
Let's consider the differences betwixt these two similarly named chemical concepts: molarity and molality. We hope that after reading this paragraph, you volition take no doubts regarding this topic.
Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the amount of substance per unit volume of solution, molality defines the concentration as the amount of substance per unit mass of the solvent. In other words, molality is the number of moles of solute (dissolved cloth) per kilogram of solvent (where the solute is dissolved in).
It is possible to recalculate from molarity to molality and vice versa. To make this shift, use the formula beneath:
molarity = (molality * mass_density_of_the_solution) / (1 + (molality * molar_mass_of_the_solute))
In this molarity vs molality table, yous can find all main differences between these two terms:
| Molarity | Molality | |
|---|---|---|
| Definition | Amount of substance (in moles) divided by the book (in litres) of the solution | Amount of substance (in moles) divided past the mass (in kg) of the solvent |
| Symbol | Grand | thousand or b |
| Unit | mol/50 | mol/kg |
| [Temperature](calc:206) and pressure level | Dependent | Independent |
| Usage | More pop, practical to employ in the lab, faster and easier | Accurate simply rarely used |
Molar solution – life examples
As you already know, mixtures and solutions e'er surround u.s., and they are a permanent role of the environment. In the table beneath, you tin detect the list of orders of magnitude for molar concentration, with examples taken from the natural environs.
| Molarity | SI prefix | Value | Particular |
|---|---|---|---|
| 10⁻¹⁵ | fM | 2 fM | Bacteria in surface seawater (1×x⁹/L) |
| 10⁻¹⁴ | – | 50–100 fM | [Gold](calc:531) in seawater |
| 10⁻¹² | pM | seven.51–ix.80 pM | [Normal](calc:472) range for erythrocytes in blood in an adult male |
| ten⁻⁷ | – | 101 nM | Hydronium and hydroxide ions in pure water at 25 °C |
| 10⁻⁴ | – | 180–480 µM | Normal range for [uric acid](calc:755) in [claret](calc:760) |
| ten⁻³ | mM | 7.viii mM | Upper jump for salubrious [claret glucose](calc:737) ii hours afterwards eating |
| ten⁻² | cM | 44.6 mM | Pure [ideal gas](calc:435) at 0 °C and 101.325 kPa |
| 10⁻¹ | dM | 140 mM | [Sodium ions in blood plasma](calc:822) |
| 10² | hM | 118.viii G | Pure osmium at xx °C (22.587 g/cm³) |
| ten⁴ | hM | 24 kM | [Helium](calc:975) in the solar cadre (150 g/cm³ * 65%) |
Determining the molar concentration by titration
Titration is a technique with which you tin discover the concentration of an unknown solution, based on its chemical reaction with a solution with a known concentration. This process is based on adding the titrant (with a known concentration & volume) to a known quantity of the unknown solution (the analyte) till the reaction is complete. You lot tin then determine the concentration of the analyte past measuring the volume of titrant used.
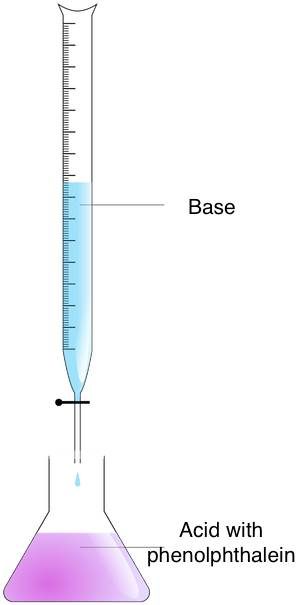
Follow these steps to find the molarity of an unknown solution with the titration method:
- Prepare the concentrations – Put the analyte in an Erlenmeyer flask and the titrant in a burette.
- Mix the concentrations – Add the titrant to the analyte until the endpoint is reached. You tin find this moment by observing the color change. Use the acid-base of operations indicator for this purpose. If you have used phenolphthalein, yous will notice a color alter from pinkish to colorless.
- Calculate the molarity – Use the titration formula. If the titrant to analyte ratio is 1:1, use the equation:
acid_molarity * acid_volume = molarity_of_base * volume_of_base.
For ratios other than 1:i, you lot demand to modify the formula.
Example: 35 ml of 1.25 M HCl acid is needed to titrate a 25 ml solution of NaOH. In that instance, you tin use the one:1 formula because ane mole of HCl reacts with one mole of NaOH. Then, multiply the molarity of the acrid by the volume of the acid – ane.25 * 35 = 43.75 and the result, past the book of the base. The molarity of the base equals 43.75 / 25 = 1.75 Grand.
You can besides determine the molar concentration of a solution by using the Beer–Lambert–Bouguer law.
FAQ
How do I calculate pH from molarity?
- Calculate the concentration of the acrid/alkaline component of your solution.
- Calculate the concentration of H+ or OH- in your solution if your solution is acidic or element of group i, respectively.
- Work out -log[H+] for acidic solutions. The result is pH.
- For alkaline metal solutions, find -log[OH-] and subtract it from 14.
How do you lot make a molar solution?
- Find the molecular weight of the substance yous'd similar to make a tooth solution of in g/mol.
- Multiply the molecular weight of the substance by the number of moles you lot wish to take, which in this case is 1.
- Weigh out the number of grams you calculated in step 2 of your substance and place it in a container.
- Measure out 1 liter of your called solvent and add it to the same container. You lot now have a molar solution.
What is molar book?
Molar book is the book that one mole of a substance takes up at a particular temperature and pressure level. It is found by dividing the molar mass by the substance's density at that temperature and force per unit area.
How exercise I find moles from molarity?
- Find the molarity and book of your solution.
- Make sure that the units for the book are the same equally for the book part of the molarity (e.1000., mL and mol/mL).
- Multiply the volume by the molarity. This is the number of moles present.
Is molarity the same as concentration?
Molarity is not the same equally concentration, although they are very like. Concentration is a measure of how many moles of a substance are dissolved in an amount of liquid, and can take any volume units. Molarity is a type of concentration, specifically moles per liter of solution.
How do you lot make a molar solution?
- Find the molecular weight of the substance yous'd similar to make a molar solution of in g/mol.
- Multiply the molecular weight of the substance by the number of moles you wish to have, which in this case is i.
- Weigh out the number of grams you calculated in footstep 2 of your substance and place it in a container.
- Measure out out 1 liter of your chosen solvent and add information technology to the same container. Y'all now take a molar solution.
What is the molarity of h2o?
Water has a molarity of 55.5 Yard. one liter of water weighs 1000 thousand, and, as molarity is the number of moles per liter; finding the molarity of h2o is the aforementioned as finding the number of moles of water in 1000 g. We therefore divide the weight past the molar mass to get moles, chiliad / 18.02 = 55.v M.
Why do we use molarity?
Molarity is a helpful measure to use when discussing concentration. As concentration has a big range of sizes of units, from nanogram per milliliter to ton per gallon, it is easier to have a known metric for quick comparing of concentrations without having to bargain with conversions. This is molarity (M), which is moles per liter.
Source: https://www.omnicalculator.com/chemistry/molarity
Posted by: farrellsymeave.blogspot.com


0 Response to "How To Find Moles With Molarity And Volume"
Post a Comment